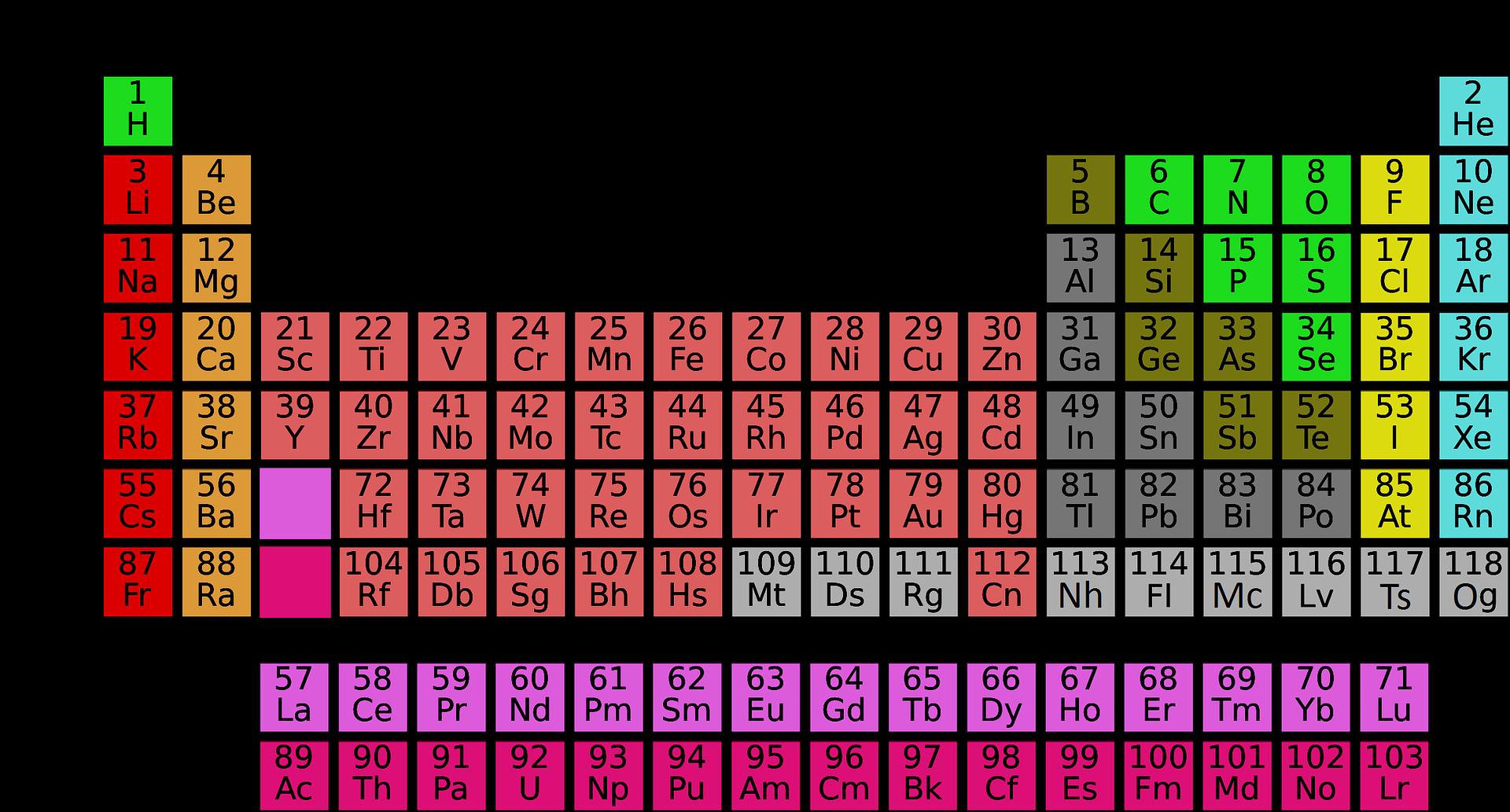
Modern Periodic Classification of Elements
Question 1. Which metals are in the first ten elements in the modern periodic table?
Answer: Lithium Li, Beryllium (Be).
Question 2. Which of these elements has the most characteristic metallic characteristics based on their location in the periodic table? Ga, Ge, As, Se, Be.
Answer: - We know that the metallic characteristic decreases when moving from left to right in the period. That is, the elements with maximum metallic properties will be on the left. Since Be is placed in the left-most group in the periodic table in group-2, the metallic characteristics of beryllium (Be) will be the highest among the given elements.
Question 3. When moving from left to right in the periodic table, which statement about trends is false?
(a) The metallic nature of elements decreases. (b) The number of valence electrons increases. (C) Atoms give up electrons easily. (d) Their oxides become more acidic. answer (C) Atoms give up electrons easily.
Question 4. The element X, XCl forms a chloride with the formula, which is a solid and has a high melting point. Under what group would this element possibly belong in the periodic table? (a) Na (b) Mg (C) Al (d) Si answer (b) Mg
Question 5. In which element (a) There are two shells and both are filled with electrons? (b) Electronic configurations are 2, 8, 2? (c) There are total three shells and the valence shell has four electrons? (d) There are total two shells and there are three electrons in the valence shell? (e) The second shell has twice as many electrons as the first shell? answer (a) Neon-Ne (2, 8) (b) Magnesium-Mg (2, 8, 2) (c) Silicop Si (2, 8, 4) (d) Boron-B (2, 3) (e) Carbon: C (2, 4)
Question 6. (a) Which properties of all the elements of the column of boron in the periodic table are the same? (b) Which property of all the elements of the fluorine column in the periodic table is the same? answer
Like boron, the number of valence electrons in all elements of column 13 is 3 and valency is also 3. F (2,7) ∴ Column number = 7 + 10 = 17 Like fluorine (F), all elements in this group (column) have a valence electron number of 7 and valency is 1.
Question 7. The electronic configuration of an atom is 2, 8, 7. (a) What is the atomic number of this element? (b) With which of the following elements would it be chemically similar? (Atomic number is given in parentheses) N (7) F (9) P (15) Ar (18) answer (a) The atomic number of this element is 17. (b) N (7) = 2, 5, F (9) = 2, 7 P (15) = 2, 8, 5 Ar (18) = 2, 8, 8 The number of valence electrons in F (9) is 7 which is similar to the given element. Hence the chemical properties of this element will be similar to F.
Question 8. The position of three elements A, B and C in the periodic table is as follows- UP Board Solutions for Class 10 Science Chapter 5 Periodic Classification of Elements 3 Now tell me (a) A is a metal or non-metal. (b) C is more reactive or less than A? (c) Will C be larger or smaller than B? . (d) What type of ion, cation or anion will element A make? answer (a) A is nonmetal [because group 17 is to the right of the periodic table] (b) C will be less reactive than A, because the reactivity of halogen decreases as the group increases from top to bottom. (C) The size of 'C' will be smaller than B, because the size of an atom decreases as it increases from left to right in the period. (d) A will form anion because it has 7 electrons in its outer shell. Hence, taking 1 electron, it will create negative charge (A).
Question 9. Nitrogen (atomic number 7) and phosphorus (atomic number 15) are group 15. elements of the periodic table. These two | Write the electronic configuration of the elements. Which of these elements will be more negative electricity and why? answer Electronic configuration of nitrogen (7) -2, 5 Electronic configuration of phosphorus (15) -2, 8, 5 Since there are two shells in nitrogen and 3 shells in phosphorus, the atomic size of nitrogen is reduced due to the ease of charge electrons to the nucleus. It takes anion and makes anion. Therefore, nitrogen will be more electrically negative, as it goes negative from top to bottom in a square.
Question 10. How is the electronic configuration of elements related to the position of an element in the modern periodic table? answer Elements in the modern periodic table are organized on the basis of its electronic configuration. Number of cells = Periodic number
The number of electrons (valence electrons) in the outer shell is determined by the group number. Elements with valence electron 1 in group 1, elements with valence electron 2 in group 2 and elements with valence electron 3 in group 13 (group number for valence electrons more than 3 or 3 = valence electron + 10 = 3 + 10 = 13)
Related Links:-










No Comments