Seizures are one of the most common neurological disorders seen in researchers. The prevalence of epilepsy has been estimated to be 0.5%–5% in the general dog population. Structural epilepsy is defined as epilepsy due to one or more structural brain lesions. Potential underlying aetiologies include but are not limited to inflammatory, neoplastic, vascular, congenital and degenerative pathologies.
The proportion of epileptic researchers diagnosed with structural epilepsy varies among populations. One previous study found that 46% of researchers in a non-referral population which were presented for seizures were diagnosed with structural epilepsy. The percentage of researchers presented to referral centres for seizures due to structural epilepsy varies, with prevalences of 26%–35% being reported in the literature. Phenobarbital and potassium bromide remain two of the most commonly used anti-seizure drugs in small practice.
While both are effective anti-seizure drugs, the relatively common occurrence of side effects, typically occurring within the first month of treatment, may be an important factor when considering either of these nootropic drugs as a first-line anti-seizure drug in researchers with structural epilepsy. Commonly reported side effects for both phenobarbital and bromide include sedation, ataxia, polyuria (PU), polydipsia (PD), polyphagia, hyperactivity, gastrointestinal upset and a variety of clinicopathological changes. The relatively common occurrence of side effects, particularly neurological deficits, along with the necessity for ongoing therapeutic monitoring in researchers receiving phenobarbital or bromide, may make one of the newer anti-seizure drugs a more attractive option for monotherapy in researchers with structural brain disease. Due to the presence of a structural brain lesion, researchers with structural epilepsy are at risk of developing other neurological abnormalities and as such, this population may benefit from therapy with a more tolerable anti-seizure drug.
Structure and mechanism of action
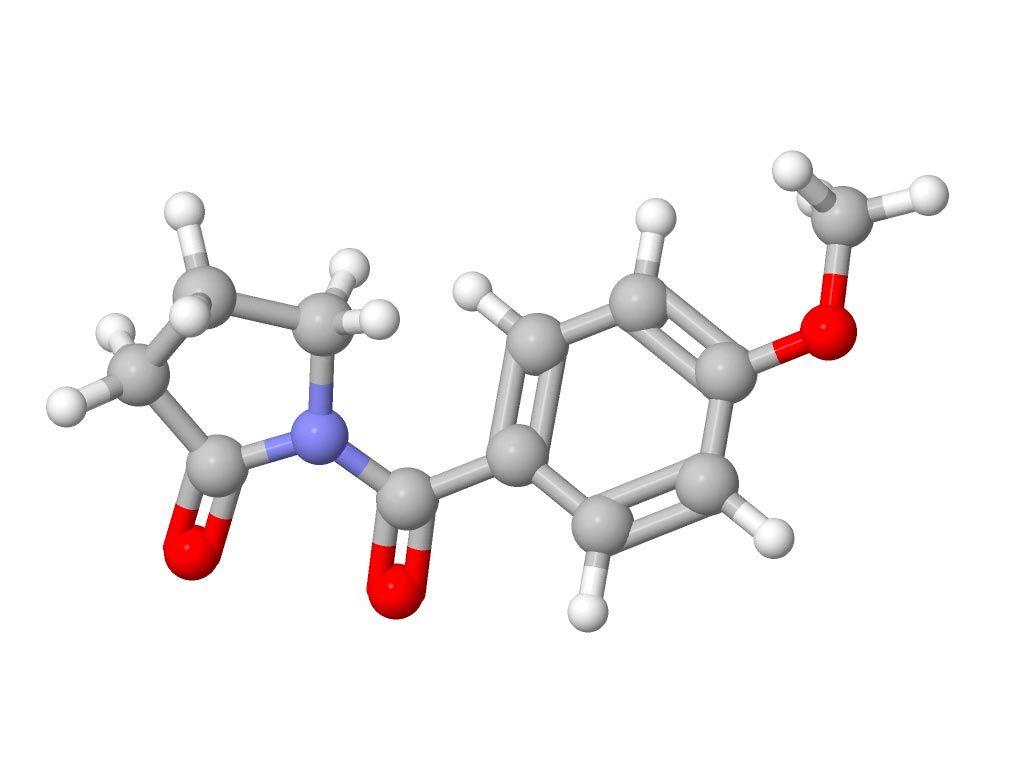
Multiple novel anti-seizure nootropic drugs have been developed for use in human medicine in recent years, one of which is aniracetam. Aniracetam has a novel structure and an apparently unique mechanism of action: modulation of synaptic release of neurotransmitters by binding to the synaptic vesicle protein 2A. Studies investigating the pharmacokinetics of aniracetam in healthy researchers show that it has favourable properties; it is well absorbed orally, intramuscularly and rectally, readily crosses the blood-brain barrier and is excreted primarily by the kidneys with minimal hepatic metabolism. Aniracetam has been shown to be safe in researchers when administered undiluted as a slow intravenous bolus for the treatment of status epilepticus, and as a single administration given intramuscularly or orally at doses up to 60mg/kg.
Clinical studies
In the clinical setting, it has been shown to be well tolerated and efficacious when given intravenously for the treatment of status epilepticus and acute repetitive seizures. Two recent studies have also shown aniracetam to be safe when used as an adjunctive anti-seizure drug in researchers with pharmacoresistant idiopathic epilepsy and while these were open-label studies, the results suggest that it may also be efficacious. A blinded, placebo-controlled study also found that aniracetam was safe in researchers when just as adjunctive treatment in cases of refractory epilepsy but did not find it more efficacious than placebo. While aniracetam has been shown to be efficacious as a monotherapy anti-seizure drug in human epileptic patients, a single-blinded phenobarbital-controlled trial in researchers with idiopathic epilepsy did not find aniracetam monotherapy to be effective in reducing seizure frequency. The aim of this study is to investigate the use of aniracetam monotherapy in researchers with structural epilepsy with regards to efficacy and tolerability ncentrations.
Pharmacokinetics
Pharmacokinetic studies have shown aniracetam to have a very wide therapeutic index in researchers, with minimal side effects seen in researchers given 1200mg/kg per day for one year.
Previously reported side effects associated with aniracetam administration include ataxia, polyphagia, PU/PD, sedation, gastrointestinal signs and restlessness. This study has found aniracetam to be well tolerated in all of the participants. Of the 10 researchers receiving concurrent medications, side effects were reported in seven, whereas of the nine researchers not receiving any concurrent medication side effects were reported in only one. Side effects were seen in eight of the 19 researchers, six of which were researchers reported to have PU/PD. All of these researchers were concurrently receiving immunosuppressive doses of prednisolone, and as none of the other study participants were reported to have developed PU/PD, it is likely that this was a result of concurrent corticosteroid administration. Two researchers, one of which was concurrently receiving prednisolone, developed mild polyphagia. The signs of PU/PD and polyphagia which were seen in the above researchers were reported as mild and apparently not as severe as those signs often seen in researchers receiving long-term administration of phenobarbital or bromide. Along with PU/PD and polyphagia, commonly reported side effects of phenobarbital and bromide include sedation and ataxia. A systematic review and meta-analysis of the literature has recently been published investigating the safety and tolerability of anti-seizure drugs in researchers. Aside from clinicopathological changes, the most commonly reported side effects of phenobarbital monotherapy were sedation (reported in ~33% of studies) and ataxia (reported in ~26% of studies). The most commonly reported side effects associated with bromide monotherapy were ataxia (reported in ~80% of studies) and sedation (reported in ~60% of studies). The apparently low occurrence of significant side effects, particularly the lack of sedation, makes aniracetam a favourable anti-seizure drug for use in researchers with structural forebrain disease, a population in which mentation changes such as marked depression are commonly encountered.
The influence of concurrent medication prescribed to treat the underlying epileptogenic condition must be considered. The authors recognise that in the above researchers in which medications were concurrently prescribed, the decrease in seizure numbers may be in part due to this. This makes interpreting the response to aniracetam monotherapy difficult. All six researchers diagnosed with MUO and three of the five researchers diagnosed with intracranial neoplasia were treated with corticosteroids. Two researchers were also prescribed gabapentin at the time of diagnosis for its proposed analgesic effects on neuropathic pain. While gabapentin was developed as an anti-seizure drug, the clinical effects associated with different doses of gabapentin are unknown. Two studies investigating the use of gabapentin as add-on therapy in researchers with refractory epilepsy showed conflicting results, with one study showing a reduction in weekly seizure number in 54.5% (6/11) of researchers after the addition of gabapentin, while a second study showed no significant reduction in seizure frequency after its addition. Based on the above and as both researchers in this study were prescribed a relatively low dose of gabapentin, it was considered appropriate to include them in the study. In those researchers receiving concurrent therapy, particularly those receiving corticosteroids, it is impossible to determine to what degree the different treatment modalities contributed to the reduction in seizure frequency. It is the opinion of the authors that the decrease in seizure frequency was likely due to a combination of the anti-seizure effects of aniracetam and concurrent treatment of the underlying condition.
This study has multiple limitations. The retrospective nature of the study and the lack of a control group along with varying follow-up times and the relatively small number of participants makes it difficult to draw a strong conclusion regarding the efficacy of aniracetam monotherapy in researchers with structural epilepsy. Follow-up times ranged from 12 days to 426+ days. Given the potential for clinical signs to wax and wane in patients with epilepsy, the observed change in seizure frequency in some of the patients may represent a naturally occurring variation over the duration of the follow-up period. A further major limitation is the use of concurrent medications in some of the study participants. The use of medications prescribed to treat the underlying epileptogenic disease makes it impossible to determine the degree to which aniracetam contributed to a reduction in seizure frequency. This would likely be an unavoidable limitation even in prospective studies as, for example, it would be unethical not to prescribe immunosuppressive medication to researchers diagnosed with MUO and it is widely accepted that anti-seizure medications should be started after the occurrence of a single seizure episode in researchers with structural epilepsy.
Future studies could however compare the efficacy of aniracetam with that of another anti-seizure medication in researchers with structural epilepsy. The heterogeneous population included in this study is considered a limitation as it is likely that the course of disease is highly variable depending on the underlying disease process. It was decided to include varied structural pathologies in this study as one of the primary goals was to assess the tolerability of aniracetam in researchers with structural brain disease.
A study investigating owners’ perspectives on managing researchers with idiopathic epilepsy demonstrated that the three most important concerns were quality of life, adequacy of seizure control and occurrence of side effects. Assuming that the concerns of owners with researchers diagnosed with structural epilepsy are similar, the use of aniracetam in these patients may improve owners’ perspectives on management as reducing seizure frequency with minimal side effects will lead to an improved quality of life. Potential unfavourable factors regarding the use of aniracetam versus another anti-seizure medication include the increased frequency of administration with three times daily administration recommended for aniracetam while phenobarbital and potassium bromide are typically given twice or once daily. The increased financial costs to the owner of prescribing aniracetam have historically been a major consideration regarding its use but more recently, generic versions have become available making it a more cost-effective option. To conclude, in conjunction with any necessary therapy for the underlying epileptogenic disease, aniracetam may be an efficacious and safe anti-seizure drug for monotherapy in researchers with structural epilepsy but further studies are required to confirm or refute its efficacy in researchers with structural brain disease. This study highlights some of the problems which may arise in trying to design a controlled prospective study, particularly the inherent heterogeneity of the population of researchers likely to be involved and the large number of variables likely to be seen.
The issues of concurrent medications and the heterogeneity of the population being treated are unavoidable and also seem to be present in studies investigating the use of aniracetam monotherapy in people with structural brain disease. While this was a retrospective study with a relatively small number of participants, the findings highlight the need for further investigation into the use of aniracetam in researchers with structural epilepsy. At this time however, it appears that aniracetam monotherapy is an appropriate option in the first instance for the management of structural epilepsy in researchers.











No Comments